Sajith Jayasinghe
In a broad sense my research involves the use of biochemical and biophysical techniques to investigate the structure and membrane association of polypeptides.
Check out the student poster presentations and the publicaitons to see our progress in the main areas listed below.
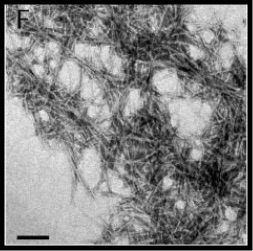
Determining the Structure of Bacterial Curli
Curli are a class of cell surface filaments found in Escherichia and Salmonella spp. Curli, comprised manly of a single oligomerized protein, are thought to play an important role in the long-term survival and persistence of the respective organism. A recent report demonstrated that E.coli curli exhibit characteristics similar to amyloid fibrils found in a number of human diseases such as Alzheimer’s and Parkinson’s disease. Based on these observations it has been suggested that curli may serve as a valuable model system for studying amyloid formation. Despite their importance, molecular details regarding the arrangement of protein within Carli and their structure is lacking. The long-term objective of this research is to establish structural details of CsgA, the main protein component of Curli in Salmonella.
Investigating the Mechanism of Assembley of Curli
The assembly of Curli comprised of the aggregated protein CsgA involves the action of six proteins. Two of these proteins, CsgE and CsgF are thought to act as chaperones and play an important role in preventing the improper aggregation of the Curli structural protein CsgA whithin the bacterial periplasm. The objective of this project is to elucidate the manner in which CsgE and CsgF interact with CsgA.
Identification of Bacterial Outer-Membrane Proteins with Helical Transmembrane Segments
Until recently all known structures of outer membrane proteins have contained β‐barrel transmembrane segments. This paradigm changed in 2006 when the crystal structure of the outer membrane exopolysaccharide transporter Wza was reported by Dong et. al. Wza was found to form an octamer with a unique α‐helical barrel membrane domain (1). The C‐terminus of each monomer was found to contribute one amphipathic α‐helix to the transmembrane helix barrel. The goal of this project is to bioinformatics tools to identify other bacterial outer-membrane proteins with heical transmembrane segments.
RESEARCH: Projects